Reusing waste materials is a key component of developing successful circular economies, and one that is working well is the reuse of steel slag. Slag from blast furnaces has been used in construction since the 1800s. Slag has been used since then in road construction, for railroad ballast, and as aggregate in concrete. Slag can be of different types with different compositions, depending on the process that produced it. Wikipedia gives a general definition of slag as a:
“…by-product or co-product of smelting (pyrometallurgical)
ores and recycled metals depending on the type of material being produced. Slag
is mainly a mixture of metal oxides and silicon dioxide. Broadly, it can be
classified as ferrous (co-products of processing iron and steel), ferroalloy (a
by-product of ferroalloy production) or non-ferrous/base metals (by-products of
recovering non-ferrous materials like copper, nickel, zinc and phosphorus).”
Steel slag has been used for
decades in Japan for road construction. It can sequester carbon through the
chemical process of carbonation if exposed to an oxygenated environment. It has
been proposed for use in green buildings, coastal protection, and agriculture. It
is made of calcium, magnesium, and silicon compounds. In the
past, it was discarded in landfills.
There are some potential
environmental impacts, including leaching of toxic heavy metals, such as
vanadium and chromium, which are often trace elements in the slag. A 2007 study
in the Journal of Hazardous Materials showed that the amounts of chromium and
vanadium that leach out over time depend on the chemical forms of these
materials in the slag. The study showed that the form of chromium (Cr) in Basic
Oxygen Furnace (BOF) steel slag is less mobile and in a less toxic form, but
vanadium is mobile and readily leached out in a toxic form.
“X-ray absorption near-edge
structure (XANES) spectroscopy indicates that Cr is present in the less mobile
and less toxic trivalent form and that its speciation does not evolve during
leaching. On the contrary, V which is predominantly present in the 4+ oxidation
state seems to become oxidized to the pentavalent form (the most toxic form)
during leaching.”
Non-ferrous slags tend to have higher concentrations of
toxic heavy metals.
Construction and Concrete
Slag has long been used in
road construction “(e.g. asphaltic or unbound layer) due to its very
high stability and superior skid and wear resistance.” Granulated
blast-furnace slag is ground into a powder for use in concrete. According to
Wikipedia:
“Ground granulated blast-furnace slag (GGBS or GGBFS) is
obtained by quenching molten iron slag (a by-product of iron and steel-making)
from a blast furnace in water or steam, to produce a glassy, granular product
that is then dried and ground into a fine powder. Ground granulated blast
furnace slag is a latent hydraulic binder forming calcium silicate hydrates
(C-S-H) after contact with water. It is a strength-enhancing compound improving
the durability of concrete. It is a component of metallurgic cement (CEM III in
the European norm EN 197). Its main advantage is its slow release of hydration
heat, allowing limitation of the temperature increase in massive concrete
components and structures during cement setting and concrete curing, or to cast
concrete during hot summer.”
The ground granulated blast-furnace slag (GGBS) is used to
make slag cement, which improves the durability of the concrete. GGBS cement
sets more slowly than concrete made with Portland cement, but it gains strength
over time and offers other advantages, including improved resistance to
alkali–silica reaction (ASR), which can damage concrete.
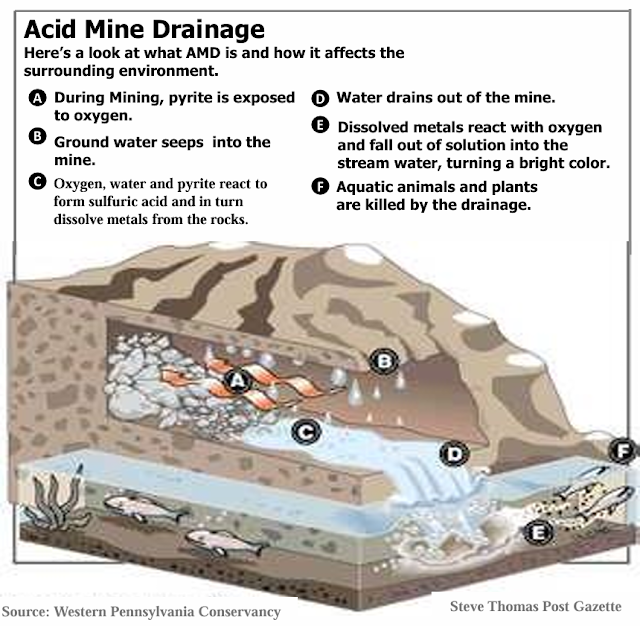
Acid Mine Drainage Treatment and Wastewater Treatment
A 2014 paper in Chemical
Engineering Journal monitored the performance of steel slag leach beds in acid
mine drainage treatment in Southeastern Ohio. Steel slag leach bed treatment is
common here in my region. Steel slag produces alkalinity, which can neutralize
acidic acid mine drainage and precipitate metals.
“Steel slag leach beds (SLBs) are a newer and
potentially promising treatment method for AMD-affected waterways. Steel slag,
a waste product from steel manufacturing, contains high concentrations of
readily dissolvable alkalinity on its surface. The alkalinity is present
primarily as Ca(OH)2 and Ca-(Fe)-silicates.”
The biggest problem with SLBs is that calcium carbonate
precipitates on the slag surfaces, effectively blocking or clogging the
treatment process. Piping can be affected as well. Thus, the slag needs to be
replaced fairly often as the ability to neutralize the acidic waters is
degraded.
One of the major advantages
of SLBs is the low cost of slag, $10-15 per ton, compared to lime (CaO) and
limestone (CaCl), which are $35 per ton, according to a 2021 presentation by
the National Slag Association. SLBs produce less sludge than lime and limestone
leach beds, which means sludge disposal costs are lower.
Agriculture
Slag, as magnesium and
calcium silicates, has the ability to provide alkalinity to acid soils, raising
the pH of the soil. Other benefits of steel slag include neutralization of Al3+
toxicity in acid soils and increased nutrient content, such as phosphorus,
calcium, magnesium, some micronutrients, and silicon. Silicon fertilization is
discussed below from the journal Recovery and Utilization of Metallurgical
Solid Waste:
“Slag application favors the increase of pH and the
availability of nutrients such as Ca, Mg, and Si in the soil, which leads to
the increase in the absorption of these elements by the plant, favoring the
growth and yield of the crops. Slags application may supply silicon which is
considered a beneficial element to plants. Silicon may bring benefits to plants
such as reduction of foliar diseases; improvement in pest control;
increase in photosynthetic capacity due to the silicon benefit to the
architectural activity of the plant, leaving the leaves more upright; and
improvement in the use of water by the plant. Si may also influence the uptake
and translocation of various macro- and micronutrients and increase plant
tolerance to excess of Mn and Fe and Zn, Al, and Cd.”’
Carbon Sequestration
Among industrial wastes,
slags have the highest potential for carbonation, the chemical uptake of
atmospheric CO2. A March 2024 paper in the Journal of CO2 Utilization explores
steel slag carbonation for carbon sequestration potential. The process of
carbonation in the steel and concrete industries has significant carbon
sequestration potential. The abstract and some figures from the paper are given
below.
The paper’s conclusion notes
the complexity of carbonation reactions and rates of reaction influenced by
factors such as temperature and pressure. They also note that carbonation has
great potential in these industries:
“The carbonation of steel slag holds great promise for
achieving carbon neutrality ambitions, not only in the steel industry, but also
in the cement and concrete industry which is another hard-to-abate sector. As
such, the value-added use of carbonated steel slag can contribute to fostering
waste-to-resource economy and enabling faster attainment of sustainable
development goals. Continued research and collaboration among scientists,
engineers, and policymakers are crucial for advancing this field and realizing
its full potential. By harnessing the massive potential for carbonation, we can
transform steel slag from a waste product into a valuable resource,
contributing to a greener future and a more sustainable steel sector.”
A January 2025 paper in
Fundamental Research explores different methods of steel slag-based carbon
sequestration, including direct carbonation, direct gas-solid carbonation,
direct aqueous carbonation, indirect acidic solution, and indirect aluminum
salt solution. Several of these processes are being explored in the lab phase,
but could be tested as pilot demonstrations at some point. The paper’s
conclusion is given below, followed by a table of the CO2 sequestration potential of electric arc furnace (EAF) slag and basic oxygen furnace (BOF) slag.
“In this study, the research and development on CO2
sequestration using steel slag (SS) was summarized. The SS-based carbon capture
and storage (SS-CCU) process is divided into direct and indirect carbonation.
The direct SS-based carbonation process is considered as an economical method
because of the involvement of cost-effective raw materials and simple
equipment. However, the product layer during the carbonation process greatly
limits the further enhancement of conversion degree of Ca. For the indirect carbon
capture process, the Slag2PCC process is a promising approach to achieve the
dual goals of CO2 sequestration and value addition of SS. Future studies on
improvement of the process may aid in increasing the selective extraction of
calcium and promoting the commercial application of carbonated products. SS is
a mixture of numerous types of minerals; therefore, some Ca and Mg enriched in
refractory minerals affect the carbonation efficiency. Thus, the SS carbonation
efficiency may be improved by enriching Ca and Mg into the high-reactive phase
through controlling the crystallization process of the molten slag. Finally,
the treatment of end products and solid residues generated by the SS-CCU
process and environmental footprint, and risk assessments undeniably need
further systematic explorations for which life cycle assessment is a suitable
quantitative and standardized tool.”
References:
The
Steel Slag Secret: How a Waste Material Is Reinforcing Roads and Fighting
Climate Change. Maria Faith Saligumba. Discover Wild Science. April 2025. The Steel Slag Secret: How a Waste
Material Is Reinforcing Roads and Fighting Climate Change
Performance
of steel slag leach beds in acid mine drainage treatment. Elaine R. Goetz and R.
Guy Riefle. Chemical Engineering Journal. Volume 240, 15 March 2014, Pages
579-588. Performance of steel slag leach beds
in acid mine drainage treatment - ScienceDirect
Environmental
impacts of steel slag reused in road construction: A crystallographic and
molecular (XANES) approach. Perrine Chaurand, Jerome Rose, Valérie Briois, Luca
Olivi, Jean-Louis Hazemann, Olivier Proux, Jérémie Domas, and Jean-Yves Bottero.
Journal of Hazardous Materials. Volume 139, Issue 3, 31 January 2007, Pages
537-542. Environmental impacts of steel slag
reused in road construction: A crystallographic and molecular (XANES) approach
- ScienceDirect
Utilization
of Steel Slag to Remediate Acid Mine Drainage. National Slag Association.
August 2021. UTILIZATION OF STEEL SLAG TO
REMEDIATE ACID MINE DRAINAGE
Research
progress of steel slag-based carbon sequestration. Qing Zhao, Chengjun Liu, Xiaohui
Mei, Henrik Saxén, and Ron Zevenhoven. Fundamental Research. Volume 5, Issue 1,
January 2025, Pages 282-287. Research
progress of steel slag-based carbon sequestration - ScienceDirect
Carbon
dioxide sequestration through steel slag carbonation: Review of mechanisms,
process parameters, and cleaner upcycling pathways. Christopher DiGiovanni,
Ousmane A. Hisseine, and Adedapo Noah Awolayo. Journal of CO2 Utilization. Volume
81, March 2024, 102736. Carbon
dioxide sequestration through steel slag carbonation: Review of mechanisms,
process parameters, and cleaner upcycling pathways - ScienceDirect
Slag.
Wikipedia. Slag - Wikipedia
Ground
granulated blast-furnace slag. Wikipedia. Ground
granulated blast-furnace slag - Wikipedia
The
Comprehensive Utilization of Steel Slag in Agricultural Soils. Angélica
Cristina Fernandes Deus, Rosemary Marques de Almeida Bertani, Guilherme
Constantino Meirelles, Anelisa de Aquino Vidal Lacerda Soares, Lais Lorena
Queiroz Moreira, Leonardo Theodoro Büll and Dirceu Maximino Fernandes. Recovery
and Utilization of Metallurgical Solid Waste. Edited by Yingyi Zhang. December 31,
2018. The Comprehensive
Utilization of Steel Slag in Agricultural Soils | IntechOpen
















No comments:
Post a Comment