Fuel cells convert
chemical energy into electrical energy. There are many different kinds of fuel
cells and fuel cell chemistry. A protonic ceramic fuel cell is a type of
protonic ceramic cell.
According to
Wikipedia:
“A protonic ceramic fuel cell or PCFC is a fuel cell
based around a ceramic, solid, electrolyte material as the proton conductor
from anode to cathode. These fuel cells produce electricity by removing an
electron from a hydrogen atom, pushing the charged hydrogen atom through the
ceramic membrane, and returning the electron to the hydrogen on the other side
of the ceramic membrane during a reaction with oxygen.”
Source: Wikipedia
Ceramic electrolytes
require high temperatures for efficiency, but lower-temperature fuel cells are
an active area of research.
“PCFCs operating at intermediate temperature of 200 - 400
degrees Celsius have been proposed for heavy duty trucking. Remote power
applications using PCFCs have been demonstrated at Canadian oil wells.”
An April 2023
paper in Electrochimica Acta explains the ongoing research with PCCs and PCFCs.
From the abstract:
“Protonic ceramic cells (PCCs), including protonic
ceramic fuel cells (PCFCs) and electrolysis cells (PCECs), are attracting
increasing attention owing to several advantages such as their low activation
energy for proton diffusion, fuel flexibility, absence of fuel dilution, and
potentially lower housing/stacking costs at intermediate operating temperatures
(400–600 °C). However, one of the major challenges for PCCs is the design and
realization of oxygen electrodes for efficient oxygen reduction and water splitting
reactions. Many research groups have devoted efforts to this research topic and
have obtained encouraging results showing improved power output and current
density in PCCs owing to the improvement of the oxygen electrode. This trend
needs to be continued to enable the commercialization of the PCC technology.
This review article describes the research progress in oxygen electrodes for
PCCs, comprehensively summarizing literature work and offering prospective
pathways for further development of high-performance oxygen electrodes.”
Researchers from
universities in South Korea have announced a breakthrough that has the potential
to double the power output of protonic ceramic fuel cells (PCCs). PCCs are like
batteries, having two electrodes and an electrolyte. I am not going to pretend
to understand the details of fuel cell chemistry or the breakthrough but I will pass on what the
scientists say. An April 2024 paper in Advanced Energy Materials explains the
breakthrough:
“The proton-conducting oxides, widely employed as
electrolytes in ceramic electrochemical cells, exhibit remarkable proton
conductivity that facilitates efficient energy conversion processes. However,
their inherent refractory nature poses a challenge in producing chemically
stoichiometric and physically dense electrolytes within devices. Here a novel
approach is presented, dual-phase reaction sintering, which can overcome the
inherent low sintering ability of the representative BaCeO3-δ‒BaZrO3-δ proton conducting oxides. This approach involves the
simultaneous transformation of a two-phase mixture (comprising fast-sintering
and slow-sintering phases) into a complete single-phase solid solution
compound, along with the densification of the electrolyte, all accomplished
within a single-step heating cycle. During the dual-phase reaction sintering
process, the grains of the fast-sintering phase experience rapid growth owing
to their intrinsic superior sintering ability. Additionally, this growth is augmented
by the Ostwald ripening behavior manifested by the smaller slow-sintering
phase. This synergistic strategy is validated using BaCe0.4Zr0.4Y0.1Yb0.1O3-δ,
and its applicability in electrochemical cells is demonstrated, resulting in a
significant enhancement in performance. These findings offer insights into
streamlining the preparation of refractory ion-conducting ceramic electrolytes
while maintaining their intrinsic properties for practical applications.”
The
dual-phase proton ceramic electrolyte produced by the low-temperature synthesis
process exhibits enhanced sintering characteristics, enabling a reduction in
the sintering temperature of conventional processes. As a result, the intrinsic
properties of the electrolyte can be realized in the device, improving cell
performance. Credit: Korea Institute of Science and Technology
PCCs can potentially be used to improve energy conversion,
resulting in better efficiency, lower cost, and lower energy use.
PCCs “utilize proton (hydrogen ion) transport instead of
oxygen ions, have emerged as next-generation energy conversion devices such as
fuel cells and electrolyzers. Unlike conventional oxygen ion-conducting
electrolytes, PCCs transport the smaller hydrogen ions, enabling higher ionic
conductivity.”
An obstacle to commercializing PCCs has been the high
sintering temperatures required to produce the electrolyte. The new research
has been able to overcome that obstacle. In order to lower that sintering temperature,
the researchers derived a new way to make the electrolyte. Typically, additives
were used to lower the sintering temperature, but those additives remain and
lower the cell’s power density. According to TechXplore:
“The research team discovered that, by synthesizing a
powder containing two different compounds through low-temperature synthesis, a
single compound with excellent sintering properties forms during the sintering
process accompanying the reaction to single phase. This allows the sintering
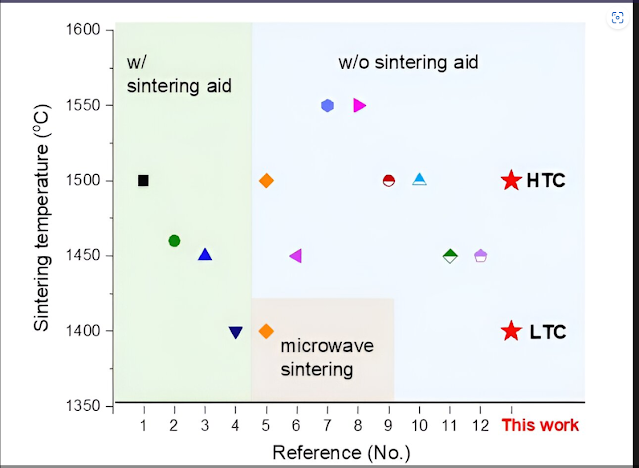
temperature to drop to 1,400°C without the need for additives.”
The
new process, which does not use sintering aids or special sintering methods,
achieved the lowest sintering temperature for electrolyte membranes. Credit:
Korea Institute of Science and Technology
Process time,
thermal stability, and performance of ceramic electrolytes are expected to be
improved with the new process.
Dr. Ji of KIST stated, "This research has resolved
the chronic sintering issues in the production of proton ceramic cells. If
large-area technology is successfully developed, it will enable efficient
energy management through green hydrogen production via electrolysis and pink
hydrogen production by utilizing waste heat from nuclear power plants.”
That means the breakthrough can also make electrolysis more
efficient, which could make green hydrogen production less expensive and help
it inch toward more widespread feasibility.
References:
Scientists
make game-changing breakthrough with next-generation power source — here's how
it works. Rick Kazmer. The Cool Down,
November 10, 2024. Scientists
make game-changing breakthrough with next-generation power source — here's how
it works
Protonic ceramic fuel cell. Wikipedia. Protonic
ceramic fuel cell - Wikipedia
Oxygen
electrodes for protonic ceramic cells. Qingjie Wang, Sandrine Ricote, and Ming
Chen. Electrochimica Acta. Volume 446, 1 April 2023, 142101. Oxygen
electrodes for protonic ceramic cells - ScienceDirect
Scientists
develop a new electrolyte synthesis method for next-generation fuel cells. National
Research Council of Science and Technology. TechXplore. October 10, 2024. Scientists
develop a new electrolyte synthesis method for next-generation fuel cells
Dual-Phase
Reaction Sintering for Overcoming the Inherent Sintering Ability of Refractory
Electrolytes in Protonic Ceramic Cells. Junseok Kim, Jiwon Yun, Wanjae Lee,
Do-Hyeong Kim, Puspendu Guha, Jin-Ha Hwang, Deok-Hwang Kwon, Sungeun Yang,
Jong-Ho Lee, Kyung Joong Yoon, Ji-Won Son, Sahn Nahm, Sihyuk Choi, and Ho-Il Ji.
Advanced Energy Materials. April 17, 2024. Dual‐Phase
Reaction Sintering for Overcoming the Inherent Sintering Ability of Refractory
Electrolytes in Protonic Ceramic Cells - Kim - 2024 - Advanced Energy Materials
- Wiley Online Library





No comments:
Post a Comment