Brine mining
refers to the extraction of minerals from brines by evaporation and/or precipitation
or by chemical, physical and/or electrical separation. Brine mining began with extracting
salt from seawater. Potassium is sometimes extracted from the bittern left over
from salt precipitation. The seawater bittern solution contains sodium, potassium,
magnesium, calcium, chloride, sulfide, iodide, and other ions that precipitate
out as compounds, as salts along with the sea salt. Bittern is one of the coagulants
used in the production of tofu. It is used in the treatment of some wastewater and
to make fertilizer. Epsom salts are another bittern precipitate. Desalination
plants process seawater to extract the salts.
The Chinese
began digging brine wells around 500 B.C., some over 330 ft deep. The wells
utilized salt-resistant bamboo for derricks, ropes, and casing. Iron wedges
pounded the bamboo into the ground by men jumping on a lever.
A January 2023
paper in Nature Water - Prospects of metal recovery from wastewater and
brine – did some technological and economic feasibility analyses of brine
and industrial wastewater mining. They concluded that initial concentrations of
the minerals in the brine and wastewaters and the cost of each mineral
commodity dictates viability. Thus, only certain brines and wastewater will be
prospective.
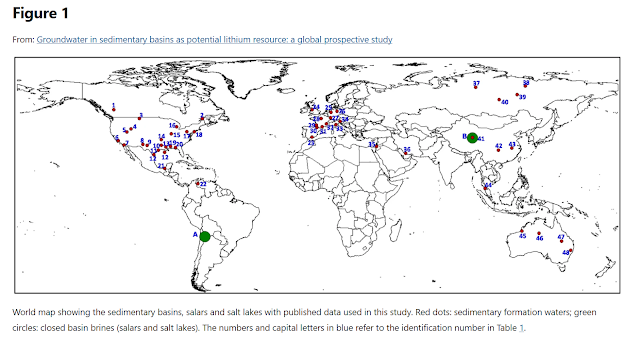
Saline Lakes, Shallow Groundwater Brines, Geothermal
Brines and Deep Brines in Sedimentary Basins
Brine is also
mined from saline lakes that have higher salinity and yield higher
concentrations of salts than seawater. The Dead Sea and the Great Salt Lake are
examples. Different saline lakes have different chemistries. Shallow
groundwater brines are associated with saline lakes or dried-up saline lakes.
Deeper geothermal brines may influence the chemistry of shallow groundwater
brines. Sodium sulfate, soda ash, salt, colloidal silica, boron, magnesium,
calcium, potassium, bromine, zinc, tungsten, and iodine are other extractable
minerals and compounds from saline lakes, shallow brines, and deeper geothermal
brines. The tables below show some of the main locations, sources, and concentrations
of mined brines.
Lithium Recovery from Ores and Brines
Lithium is produced
from lithium-rich rock from pegmatite deposits such as spodumene and from the
extraction of salts from subsurface brines. Some lithium is also expected to be
produced from clay. Lithium from rock ore is of higher concentration but is
more costly to produce because it requires digging out, crushing, heating, and
cooling. Lithium from shallow brines is concentrated through time in evaporated
ponds called salars in Chile, Argentina, and Bolivia in an area of the Andes
with very little rainfall. Brine is extracted from shallow wells and evaporated
in ponds on the surface to concentrate the mineral precipitates. When
concentrations reach suitable levels, the mineral salts are taken for further
processing. Now there are new methods to extract lithium and other minerals
from brines that do not require the very large evaporation ponds.
The economics
of lithium extraction depend on the concentrations of lithium in the brines and
ores, the cost of extraction, and the price of lithium. Lithium prices have
been high in recent years and demand is expected to stay robust in the years to
come as more EVs, grid-scale batteries, and lithium-powered products are built. The downsides of lithium extraction via solar evaporation ponds include the long
timelines (18 months), the large amount of required acreage, and the environmental
impacts.
Lithium is
further processed into metals. Via an electrolytic cell, lithium chloride (55%)
is mixed with potassium chloride (45%). This makes a molten eutectic electrolyte.
The combined chemicals increase the conductivity of the lithium and lower the
fusion temperature. When fused and electrolyzed at 840 deg F chlorine gas is
liberated, and molten lithium rises to the surface. It is collected in cast
iron and treated with paraffin to prevent oxidation. It takes about 5.3 times
as much lithium carbonate to make the same amount of lithium metal.
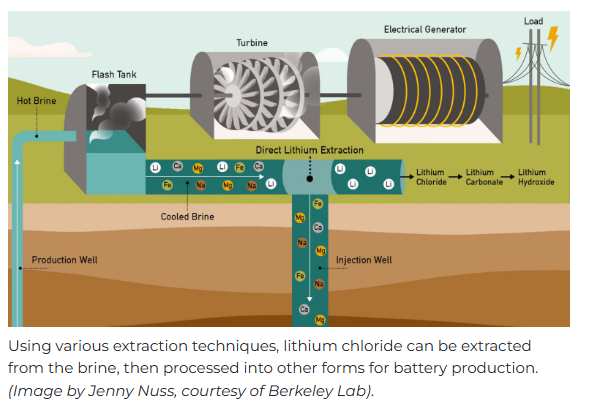
Direct Lithium Extraction (DLE) from Brines
The methods of
lithium production in the salars and solar evaporation ponds in South America
are not applicable to other places due to space requirements and environmental degradation. The South American high deserts are not populated,
have a low amount of flora and fauna, and much of the groundwater (the brine)
is already naturally contaminated and unsuitable for drinking. Direct lithium
extraction (DLE) has far less environmental impact and space requirements. The
DLE process relies on filters, membranes, ceramic beads, and other equipment to
extract lithium and other minerals. The post-extraction water is pumped into wastewater
injection wells nearby. The process resembles a wastewater treatment plant.
An article in
Civil Engineering Magazine describes it as follows: “Depending on a
particular brine’s geochemistry, various pretreatment steps are used to remove
solids, hydrogen sulfide, and other contaminants. Adsorption, ion exchange, and
solvent extraction are among the most common methods under development to
extract lithium directly from brine.” DLE methods are applicable as well to
the South American brines traditionally extracted with solar evaporation ponds
and offer a way for those companies to reduce their environmental footprint. Goldman
Sachs thinks DLE will be adopted in many of those operations in the 2025-2030
timeframe. Their market report from late April 2023 suggests that costs are
comparable between solar evaporation ponds and DLE. DLE requires higher upfront
costs but recovers much higher percentages of lithium. The ability to eliminate
many of the environmental downsides at comparable costs makes widespread
adoption likely.
A paper in Nature
Reviews Earth & Environment - Environmental impact of direct lithium
extraction from brines – noted that many environmental impact studies of
DLE thus far have not been performed on real brines. Thus, there may be some
pre-processing issues for different brine chemistries. Those often require heat
energy and have emissions. Environmental impact studies of DLE methods need to
consider the use of heat, pH adjustment, and other energy and water inputs when
doing a lifecycle analysis for each method. For instance, methods requiring
high water inputs would not be environmentally sound in arid areas. They would also
be less cost-effective.
There are
three main DLE methods in operation and two more in development. Those in operation
are adsorption, ion exchange, and solvent extraction. Those in development are membrane
separation and precipitants. High lithium demand and prices and new government
incentives for domestic critical minerals production in the U.S. have spurred
new pilot and production projects for DLE technologies.
Adsorption is
the most developed DLE method globally. This method is used in many other
industries as well. One of the advantages of adsorption is that it uses water to
yield lithium chloride and soda ash to yield lithium carbonate rather than using
reagents (acids). It produces less waste and is efficient as it extracts over
90% of the lithium. It does have high upfront costs, some process contamination
issues, can have high operating costs, and may require additional heat energy.
According to Goldman Sachs: “Ion exchange systems separate ionic contaminants from solution through a physicochemical process where undesirable ions are replaced by other ions of the same electrical charge. Essentially, the ion-exchange material acts as a sieve with an adjusted porosity that only allows lithium (and hydrogen) ions to pass through, where the ion-sieve can then be washed with an acidic solution promoting the replacement of lithium ions with hydrogen ions. Lithium recovery by ion exchange can change with a simple adjustment in pH, temperature, or stream composition (though the same goes for other lithium extraction methods), but researchers also believe this method can recover ~90% of the lithium present.” Several of the Salton Sea projects as well as Standard Lithium’s Smackover projects plan to use ion exchange DLE with some expected to commence operation in 2024. Advantages of ion exchange include simplicity of the process, low chance of process contamination, high capacity, ability to process low-concentration brines, low energy and water consumption, and likelihood of continuous operation. The downsides include high acid and base input requirements with accompanying risks, high upfront costs, and some components vulnerable to acid degradation.
Solvent
extraction utilizes a physical means or a chemical combination of a solvent (often
kerosene) and an extractant to separate out the lithium since the extractant will
select lithium over sodium and magnesium. Solvent extraction can also be used
as a post-DLE step to “polish” the lithium closer to battery quality. The
method has a high recovery rate, and low operating costs, and it eliminates one of
the steps in the other two methods. The downsides of the method are that it is
not applicable to brines with lower lithium concentrations, or those with high
concentrations of magnesium and calcium (which would require preprocessing). The
solvents are also a fire risk with high temperature brines, have corrosion
issues, and environmental transport and disposal issues. It is also an
expensive process.
Membrane
separation utilizes membranes to separate lithium and magnesium ions and may
utilize one of the following to induce the process: pressurized nanofiltration,
electric field(electrodialysis), or thermal gradient. The process is efficient with
low environmental impact but is not applicable to brines with high sodium or
potassium concentrations. It is a water-intensive process that is also
expensive.
An interesting
new membrane separation method involves using 12-Crown-4–functionalized polymer
membranes in coordination with ion binding sites to select for lithium chloride
over sodium chloride. Imbuing the polymers with interactants gives them the
ability to foster the ion transport that separates the lithium out. The sodium
ions bind to the crown ether enough to slow them down so the lithium ions
travel faster through the polymer. The researchers from the University of Texas at Austin
and the University of California, Santa Barbara think that this is a promising
method for extracting from oilfield-produced water. The table below from Goldman Sachs compares
the different brine extraction methods.
The Upper Jurassic Smackover Formation Deep
Sedimentary Basin Lithium Brine Play
Thus far, the
best brine lithium concentrations in the U.S. have been found in the Upper
Jurassic Smackover Formation which is distributed deep in the subsurface along
the Gulf Coast Basin from Eastern Texas to Western Florida. It is a deep
sedimentary basin brine developed in the past as a very productive oil &
gas reservoir in Mississippi, Louisiana, Alabama, and Southern Arkansas. The
brine production comes from zones about 7500-8500ft below the surface in Southern
Arkansas. A company called Standard Lithium has been focusing on lithium extraction
from Smackover brines for a few years now. It is probably one of the best
lithium brines outside of Chile and Argentina, according to Standard Lithium.
Just a few
weeks ago on October 10, 2023, Standard Lithium announced that from a newly
drilled well in East Texas they achieved the highest brine concentration in
North America at 663mg/L which is comparable to the South American brines. Two
samples were taken from the Upper Smackover with the avg. lithium concentration
at 638mg/L. Just a few days ago they reported that a sample from another well
measured lithium at 806mg/L which broke the record from a few weeks ago. The
average overall for their East Texas field is 644mg/L. In addition to lithium,
Standard’s East Texas wells tested at economically viable concentrations of
potassium and bromine as well. Lithium concentrations as high as 597mg/L have
been found in Southwest Arkansas.
The Smackover
formation is limestone, often sucrosic, and locally dolomitized. It overlies
the Norphlet formation and more importantly the Louann Salt formation. The Louann
Salt is the source of the minerals that migrated via faults through the
Norphlet formation into the Smackover. Authors Eva P. Moldovanyi and Lynn M.
Walter in a 1992 AAPG Bulletin article note that their regionally extensive
database of Smackover water geochemistry shows that heterogeneities in
Smackover water geochemistry occur along faults due to upward migration of
hotter fluids. “One of the most pronounced heterogeneities in water
chemistry is an H2S-rich anomaly in the center of the shelf. Enrichments in H2S
are accompanied by large increases in alkali elements (Li, K, and Rb) and B,
which could be produced by higher temperature diagenesis of clay minerals and
feldspars in deeper seated siliciclastic strata.” They also note that bromide
and chloride, concentrated by seawater evaporation, were further enriched by halite
dissolution followed by expulsion of meteoric waters along fault systems.
Looking at Standard Lithium’s Li concentration numbers in Southwest Arkansas on
a map suggests that while Li concentrations vary quite a bit, they could be
contour mapped and compared with structural geology to help determine trends. In
a 2018 thesis, Pamela Joy Daitch utilized the U.S. Geological Survey National
Produced Waters Geochemical Database to identify lithium-rich brine from wells
across the U.S. She found that the Smackover formation was by and far the main
lithium-rich formation brine in the U.S. Based on financial analysis she also determined
that the best way to develop the Smackover was to drill standalone brine wells
where concentrations are the highest. This is how Standard Lithium is
approaching the play. She also noted an economic cutoff concentration for
lithium production of about 70mg/L. This, of course, will vary depending on
lithium prices and extraction innovations. Some of the lithium projects in
Alberta, Canada are at the low end of profitability with concentrations of
about 75mg/L.
The Smackover Bromine Play
The Smackover
formation brine has long been a major source of bromine. Bromine production in
Southern Arkansas began in 1957. According to the Arkansas Dept. of Energy
& Environment: “Since 2007, all US bromine has been produced in southern
Arkansas. In 2013, 28% of the global bromine production (225,000 tonnes) in
Arkansas made the United States the second-largest producer of bromine, after
Israel. At an advertised price of US$3.50 to US$3.90 per kg, the 2013 Arkansas
production would have a value of roughly US$800 million.” Fire retardants
are the major use of Arkansas bromines, making up about half of the production.
Other uses include insect and fungus sprays, anti-knock compounds for leaded
gasoline, disinfectants, photographic preparations and chemicals, solvents,
water-treatment compounds, dyes, insulating foam, hair-care products, pharmaceuticals,
energy storage, mercury emissions reduction, reinforcing rubber materials, and
oil well-drilling fluids. Bromine is a highly corrosive liquid. It is harmful
if it contacts human skin and breathing its vapors is harmful, so safety precautions
are in place at processing plants. Albemarle Corporation and Chemtura are the
two biggest bromine producers in South Arkansas.
The Salton Sea Geothermal Brines Lithium Play in
Southern California
In
California, the geothermal brines below the evaporating Salton Sea are a great
source of lithium and other minerals. The hot brines are already being
circulated to the surface in geothermal wells to power the steam turbines of
the geothermal plants. Brine production rates are high in the Salton Sea. The high
flow rates associated with the geothermal wells mean processing rates have to
be high to keep up. This is challenging. Many DLE projects are ramping up in
the Salton Sea play. Investors include Energy ventures by Bill Gates and Jeff
Bezos, Warren Buffet’s Berkshire Hathaway, the U.S. DOE, and automaker
Stellantis. The biggest players in the Salton Sea DLE Projects are Berkshire Hathaway
Energy, EnergySource Minerals, and Controlled Thermal Resources (CTR).
The Salton Sea
is one focus for the U.S. ramp-up of domestic critical minerals production,
deemed as necessary to reduce dependence on China and to eventually ease supply
chain issues. Typically, geothermal energy powers mineral extraction, keeping
emissions low. Some CO2 is released in the steam that surfaces in the course of
geothermal energy production.
As of 2022, there were 11 geothermal power plants in the Salton Sea area, also now known as
Lithium Valley. As mentioned, the flow rates from the geothermal recycling
process are very high so the extraction methods need to be scaled up for the
higher flow rates. While the Smackover play has a much higher lithium concentration
than the Salton Sea, it may have a lower brine production rate. I am just
speculating here. The Salton Sea has the advantage that the extraction of the metals is a co-benefit to the geothermal power plants so that operation is simultaneous.
It is unlikely that existing Smackover oil and gas wells will develop mineral extraction as a co-benefit, especially since standalone brine wells have been
the preferred method for bromine recovery for many years. Berkeley Lab has been
leading the effort to quantify the Salton Sea geothermal brine resources.
Flow rates may
also affect the depletion of the brine of the desired minerals. Productivity of
wells, flow rates and recirculation rates, and geochemistry of the brine are
some of the main variables that can affect depletion. The paper about the environmental impacts of DLE from brines emphasized the importance of geochemical
heterogeneity as follows: “Knowledge of the precise number, distribution and
depths of brine and fresh water wells is vital for hydrogeological modeling of
lithium brine deposits. The distinct hydrogeology of each salar means that each
deposit should be modelled independently, and results from one exploitation
cannot be directly extrapolated to another.” This suggests that there should
be more data collected as brine samples at different depths in many more wells
to map out mineral concentrations in each zone. This data should be compared to
geology trends like faults and stratigraphy.
Brine Mining from Marcellus Shale Produced Water in
Pennsylvania
Brine mining
projects in the Marcellus Formation in Pennsylvania tap a brine with a lithium
concentration of 95mg/L on average but also leverage pre-existing oilfield
water treatment facilities where direct mineral extraction provides additional
revenue. According to Shale Directories: “Eureka Resources owns and operates
three centralized treatment/recycling facilities that process flowback/produced
waters (i.e. wastewater) from the Marcellus Shale. Two of the facilities are
located in Williamsport (Lycoming County), PA, and one in Standing Stone
Township (Bradford County), PA, near Towanda. Eureka has just announced a joint
venture to use high tech to recover lithium from the Marcellus wastewater they
process.” The lithium recovery venture was announced together with Canada’s
MGX Minerals in March 2019. The Eureka process treats about 10,000 Bbls
(420,000 gals) per day of brine to extract sodium chloride, calcium chloride, freshwater,
and lithium. MQX Minerals uses its own DLE method to extract lithium.
Together the two companies plan to install multiple lithium rapid recovery
systems at wastewater treatment facilities across the Marcellus and Utica shale
formations. Utica brine has a lower average lithium, around 70mg/L which is near
the cutoff of economic feasibility. This effort opened the gates to petrolithium, the extraction of lithium from oilfield brines. They say they can process oilfield
brines rapidly and at smaller scales as well.
In November
2020 Eureka Resources got a patent for their own lithium extraction technology
and expanded their Northeast Pennsylvania operations. Eureka reported in late
July 2023 that they successfully produced 97% pure lithium carbonate from the
Marcellus brine, in partnership with SEP Salt & Evaporation Plants Ltd.
(SEP). Apparently, they are utilizing the same methods to extract lithium as they
use to extract sodium chloride and calcium chloride. They say it will be
operational within two years, by mid-2025, which would make it the fastest-to-market lithium brine extraction technology, including DLE (it is apparently not
a DLE method as normally understood). The brine is pretreated to remove heavy
metals, ammonia, magnesium, sulfates, carbonates, and TOC through filtration, pH
adjustment and precipitation reactions. SEP employs several proprietary
extraction and recrystallization processes.
Oilfield Brines Processing. Source SEP
Paradox Basin Brine Mining Play in Utah and Colorado:
Lithium, Potash, Bromine, and Boron
The Paradox Basin is a Pennsylvanian-aged sedimentary
basin with a thick sequence of evaporites and brines that yield favorable concentrations
of minerals. It is thought to be one of the largest potential sources of potash
in America. The brines also contain commercial concentrations of lithium,
bromine, boron, and likely iodine. The depths of the brines in the Southern Natural
Gas well log and stratigraphic column (shown below) range from 5000ft to 7500ft below the surface. American Potash Corporation is exploring for brine minerals in the
Paradox Basin and expects to employ both solar evaporation ponds and direct
extraction.
Anson
Resources plans to extract lithium and bromine from Paradox Basin brines in
Utah. According to Mining Weekly: “The proposed Phase 2 expansion will
target substantial expansion in the production of lithium carbonate and
bromine, and will expand the Paradox project resources through the re-entry and
sampling of historic wells, including Mineral Canyon; Sunburst; and high-grade,
large Mississippian formations.” Thus, they plan to get more concentration
data, including for deeper Mississippian formations.
Brine Processing Plants Processing Oilfield Brines and
Desalinization Plant Effluent for Minerals Extraction, Irrigation, and Drinking
Water
A Brine processing
plant plans to extract minerals from oilfield brine and brackish brine from the
adjacent desalinization plant. This is not happening anywhere yet but a project
in El Paso, Texas, that previously failed to materialize due to financial,
investor, and engineering difficulties was purchased by a Florida company,
Critical Minerals Corporation that plans to reconfigure and run the plant. The desalinization
plant, the largest inland one in the U.S., has been in operation since 2007.
The plan is to treat the brine and extract minerals to sell to oil & gas
companies and for fertilizer. Lithium extraction is also in the works when more
lithium-rich brines can be delivered. The brine processing plant will be the first
of its kind in the country. I am not sure about the timeline or what stage of
commercialization is happening at present.
Japanese Iodine-Rich Brine and Natural Gas Play
Chile produces more
than half of the world’s iodine and Japan about 30%. Together the two countries
produce nearly 90% of the global supply. Chile’s production comes from naturally
concentrated precipitates. Japan produces iodine along with natural gas from
brines. Natural gas is dissolved in the brines in Chiba prefecture and other
areas. According to the book Iodine Chemistry and Applications: “Iodine
production in Japan occurs at the Minami-Kanto gas field near Tokyo, the
Niigata gas field, the Nakajo oil and gas field, both in Niigata Prefecture,
and the Sadowara gas field in Miyazaki Prefecture, all of which are
brine-dissolved gas fields. Although iodine is known to exist at high
concentrations in submarine sediments, the commercial production of iodine from
brine involves drilling on land. In Japan, iodine is produced from natural gas
brine by a blowing-out process and an ion-exchange process.” After
extraction, the water must be reinjected back into the ground to prevent land subsidence
due to the presence of unconsolidated sediments. This prevents the operations
from expanding processing volumes. Below is a schematic of the blowout process
for extracting iodine.
Iodine Brine Play in Pennsylvanian Morrow Sandstone in
the Anadarko Basin in Northwest Oklahoma
The U.S. is the world’s
third-largest iodine producer. The iodine in the U.S. comes from the Late Pennsylvanian-aged Morrow Sandstone in the Anadarko Basin of Northwest Oklahoma. AAPG’s Susan
Nash writes “Chile is the world’s dominant producer, where iodine is
recovered principally by heap-leaching nitrates containing iodate. In Japan’s
Chiba prefecture, iodine is recovered from natural gas brines using the blowing
out process, which involves vaporizing the iodine, then absorbed, crystallized,
and purified. In Oklahoma, the iodine is found in the Morrow sand brines. It is
also extracted via proprietary processes from the oilfield brines co-produced
with oil and gas. There are currently three operators in Oklahoma, along with
another chemical company that creates products from the brine.” The iodine-rich
brine is produced from a single paleo-valley located in the Anadarko Basin in
one specific feature, the Woodward Trench, a paleo-valley 1 to 2 miles wide in
the Morrow formation that extends 70 miles from Vici, Oklahoma, north to the
Kansas border. The source of the iodine is the Late Devonian/Early
Mississippian Woodford Shale which lies below the Morrow. Organic matter
including seaweed, brown algae, coral, and other marine life helped to
concentrate the iodine in the shale. The iodine concentration in the Mississippian
rocks of the Woodward Shale is as high as 1560 parts per million (ppm). The iodine-rich
brines occur at depths from 6000 ft to 10,000 ft below the surface. The iodine
concentration in the Morrow Sandstone is as high as 700 ppm. This is about twice as concentrated as the
Japanese brines which have average concentrations of about 160 mg/L. This maximum
concentration (700 ppm) is at least three times higher than anywhere else found
in the U.S. with the exception of newly found iodine-rich brines in the Paradox
Basin with concentrations as high as 596mg/L, almost twice that of the Morrow Sand. Nash wonders whether there are
any other paleo valleys in the Anadarko vicinity or elsewhere with iodine-rich
brine that await discovery.
The first iodine
processing plant was built in Oklahoma in 1977, based on Japanese and European
designs. Acids are used in iodine processing, which requires precautions for
handling corrosive material. Iodine prices rose sharply in 2017-2023 by 200%. Uses
for iodine include X-ray contrast (biggest use), disinfectants, liquid crystal
displays (LCDs), nutritional supplements for humans and animals, pharmaceuticals,
LEDs, and specialty chemicals. Iodine recovery from oilfield brines involves
settling tanks where the oil is skimmed off the top and chlorination of the
brine which separates out the iodine. The vaporized iodine compounds are
condensed back into a liquid or solid form through distillation or sublimation and
further purified by extraction through oxidation-reduction reactions. The
process yields flakes or pellets of 98% pure iodine.
This map does not have the 596mg/L data point in Southeast Utah Paradox Basin
Leduc Lithium Brine Play in Western Canada
E3 Lithium
reported in mid-October 2023 that they recently tested their DLE pilot project
in Alberta’s Leduc Brines. The tests evaluated lithium recovery and lithium
grade at different flow rates. The company noted that all the parameters
exceeded their expectations. Thus, they are optimistic about continuing toward
commercialization. The Leduc brines are at the lower end of lithium concentrations
for economic viability so I would guess projects in that range would be
especially sensitive to lithium prices and things like O&M costs.
Calgary, Alberta-based Volt Lithium has a pilot demo project at Rainbow Lake in northwest Alberta. They are processing oilfield brines from throughout North America. Lithium hydroxide and lithium carbonate are produced. The pilot was a success. They are now building a permanent demonstration plant. A schematic is shown below.
Extraction of Rare Earth Elements from Brines: Currently,
Not Economically Feasible
A 2017 paper On
the Extraction of Rare Earth Elements from Geothermal Brines. York R. Smith,
Pankaj Kumar, and John D. McLennan. Resources 2017, 6(3), 39, concluded
that “rare earth element extraction from geothermal fluids is technically
possible, but neither economically viable nor strategically significant at this
time.” Rare earths can far better be extracted from veins of much higher
concentration than brines. Basically, the same separation methods used for
critical minerals would be used to separate rare earth elements in brines, if
and when doing so ever becomes feasible.
Market Predictions and Incentives
Most economic
scenarios that incorporate the energy transition forecast continued increases
in demand for lithium and some of the other brine minerals. DLE as a
processing method is very desirable due to its low environmental, climate, and
land footprints. It is already modeled as cost-comparable to solar evaporation.
Thus, it seems likely that widespread adoption is likely in the next 5-10
years. The number of pilot and commercialization projects in development and
planning has been growing. Over the next 2-5 years we should get an idea of what
DLE can do to meet demand and for the U.S. perhaps ensure a more robust domestic
supply with clean energy credentials.
In the U.S.
the Critical Minerals Exploration Tax Credit (CMETC) was expanded from 15% to
30% as part of the Inflation Reduction Act. This credit includes lithium and a
few other brine minerals like zinc and magnesium. It will offset some of the
inflation and help companies commercialize their projects and operations.
References:
Brine
Mining. Wikipedia. Brine mining - Wikipedia
Bittern. Wikipedia. Bittern (salt) - Wikipedia
Standard
Lithium’s East Texas Drilling Program Delivers New Highest Confirmed Grade
Lithium Brine in North America. Standard Lithium. Press Release. October 10,
2023. Standard Lithium’s East Texas
Drilling Program Delivers New Highest Confirmed Grade Lithium Brine in North
America :: Standard Lithium Ltd. (SLI)
E3
Lithium Says Field Pilot Test Results Exceed Expectations. Hart Energy. October
18, 2023. E3 Lithium Says Field Pilot Test
Results Exceed Expectations | Hart Energy
An
Overview of Commercial Lithium Production. Terence Bell. ThoughtCo. August 21,
2020. Commercial Lithium Production and
Mining of Lithium (thoughtco.com)
New
methods could extract large lithium stores from brine. Jay Landers, Civil
Engineering Magazine. October 26, 2023. New methods could extract large
lithium stores from brine | ASCE
Standard
Lithium Delivers Highest-Ever North American Lithium Brine Grade 806 mg/L; East
Texas Asset Includes Significant Potash and Bromine Concentrations. Standard
Lithium. October 25, 2023. Standard Lithium Delivers
Highest-Ever North American Lithium Brine Grade 806 mg/L; East Texas Asset
Includes Significant Potash and Bromine Concentrations :: Standard Lithium Ltd.
(SLI)
Petrolithium:
Extracting Minerals From Petroleum Brine. Jared Lazerson, MGX Minerals. Hart
Energy. April 3, 2017. Petrolithium: Extracting Minerals
From Petroleum Brine | Hart Energy
Environmental
impact of direct lithium extraction from brines. María L. Vera, Walter R.
Torres, Claudia I. Galli, Alexandre Chagnes & Victoria Flexer. Nature
Reviews Earth & Environment volume 4, pages149–165 (2023). February 23,
2023. Environmental impact of direct
lithium extraction from brines | Nature Reviews Earth & Environment
Groundwater
in sedimentary basins as potential lithium resource: a global prospective study.
Elza J. M. Dugamin, Antonin Richard, Michel Cathelineau, Marie-Christine
Boiron, Frank Despinois & Anne Brisset. Scientific Reports volume 11,
Article number: 21091 (2021). October 26, 2021. Groundwater in sedimentary basins as
potential lithium resource: a global prospective study | Scientific Reports
(nature.com)
Prospects
of metal recovery from wastewater and brine. Ryan M. DuChanois, Nathanial J.
Cooper, Boreum Lee, Sohum K. Patel, Lauren Mazurowski, Thomas E. Graedel &
Menachem Elimelech. Nature Water volume 1, pages37–46 (2023). January 19, 2023.
Prospects of metal recovery from
wastewater and brine | Nature Water
Regional
Trends in Water Chemistry, Smackover Formation, Southwest Arkansas: Geochemical
and Physical Controls. Eva P. Moldovanyi and Lynn M. Walter. AAPG Bulletin
(1992) 76 (6): 864–894. Regional
Trends in Water Chemistry, Smackover Formation, Southwest Arkansas: Geochemical
and Physical Controls1 | AAPG Bulletin | GeoScienceWorld
Lithium
extraction from oilfield brine. Pamela Joy Daitch. Thesis Abstract, June 19,
2018. University of Texas at Austin. Lithium extraction
from oilfield brine (utexas.edu)
As
Companies Eye Massive Lithium Deposits in California’s Salton Sea, Locals
Anticipate a Mixed Bag. June Kim. Inside climate News. August 26, 2023. As
Companies Eye Massive Lithium Deposits in California’s Salton Sea, Locals
Anticipate a Mixed Bag - Inside Climate News
Lithium
recovery from shale gas produced water using solvent extraction. Eunyoung Jang, Yunjai Jang, Eunhyea
Chung. Applied Geochemistry. Volume 78, March 2017, Pages 343-350. Lithium
recovery from shale gas produced water using solvent extraction - ScienceDirect
Eureka
To Extract Lithium From Marcellus/Utica Wastewater. Shale Directories. March
2019. Eureka
to Extract Lithium from Marcellus/Utica Wastewater - Shale Directories
MGX
Minerals and Eureka Resources Announce Joint Venture to Recover Lithium from
Produced Water in Eastern United States. MGX Minerals. March 5, 2023. MGX
Minerals and Eureka Resources Announce Joint Venture to Recover Lithium from
Produced Water in Eastern United States (prnewswire.com)
Lithium
recovery from brine: Recent developments and challenges. Abdullah Khalil,
Shabin Mohammed, Raed Hashaikeh, Nidal Hilal. Desalination. Volume 528, 15
April 2022, 115611. Lithium
recovery from brine: Recent developments and challenges - ScienceDirect
Florida
firm plans to mine minerals by reviving failed El Paso brine treatment plant. Vic
Kolenc. El Paso Times. April 21, 2021. Florida
firm plans to mine brine waste by reviving El Paso plant (elpasotimes.com)
Technology
for the Recovery of Lithium from Geothermal Brines. William T. Stringfellow and
Patrick F. Dobson. Energies 2021, 14(20), 6805. October 18, 2021. Energies | Free Full-Text |
Technology for the Recovery of Lithium from Geothermal Brines (mdpi.com)
Geothermal
brines in California’s Salton Sea could be future source of lithium in the US. Valentina
Ruiz Leotaud. Mining.Com. December 12, 2021. Geothermal
brines in California’s Salton Sea could be future source of lithium in the US -
MINING.COM
Paradox
basin brine project. US. Creamer Media’s Mining Weekly. May 12, 2023. Paradox
basin brine project. US (miningweekly.com)
Lithium
& Brine Project: Paradox Basin, Utah. American Potash Corporation. Presentation.
September 2022. paradox_basin_lithium_and_brine_project_sept_2022.pdf
(americanpotash.com)
General
Information Regarding Brine in Arkansas and Geology of Brine Resources in South
Arkansas. Arkansas Dept. of Energy & Environment. Office of the State
Geologist. Brine
(Bromine) in Arkansas
Brine
Mining Iodine in Oklahoma: A Little-Known Treasure. Susan Nash, Director of
Innovation & Emerging Science and Technology / AAPG. June 11, 2023. (24)
Brine Mining Iodine in Oklahoma: A Little-Known Treasure | LinkedIn
Direct
Lithium Extraction: A potential game-changing technology. Goldman Sachs. April
27, 2023. Global
Metals & Mining Direct Lithium Extraction A potential game changing
technology (goldmansachs.com)
Iodine.
U.S. EPA. Identification
and Description of Mineral Processing Sectors and Waste Streams | US EPA
ARCHIVE DOCUMENT
Iodine.
Stanley T. Krukowski. Oklahoma Geological Survey. Mining Engineering. July 2016.
MEV68.7P54-57-Krukowski-Iodine.pdf
(ou.edu)
Engineering
Li/Na selectivity in 12-Crown-4–functionalized polymer membranes. Samuel J.
Warnock, Rahul Sujanani, Everett S. Zofchak, and Christopher M. Bates. PNAS.
September 7, 2021 118(37).
Engineering Li/Na
selectivity in 12-Crown-4–functionalized polymer membranes | PNAS
How
lithium can be efficiently extracted from oil and gas wastewater. Mining.Com.
Staff Writer | September 22, 2021. How
lithium can be efficiently extracted from oil and gas wastewater - MINING.COM
On the
Extraction of Rare Earth Elements from Geothermal Brines. York R. Smith, Pankaj
Kumar, and John D. McLennan. Resources 2017, 6(3), 39. Resources | Free Full-Text |
On the Extraction of Rare Earth Elements from Geothermal Brines (mdpi.com)
Eureka
Resources expanding operations in northeast Pennsylvania. NCPA Staff Mar 29,
2021. North Central PA. Eureka
Resources expanding operations in northeast Pennsylvania | Business |
northcentralpa.com
Eureka
Resources Successfully Produces Lithium Carbonate From Oil & Natural Gas
Brine Wastewater. July 31, 2023. Business Wire. Eureka
Resources Successfully Produces Lithium Carbonate From Oil & Natural Gas
Brine Wastewater | Business Wire
Shale
Gas Produced Water. SEP. Shale
Gas Produced Water (sepwin.ch)
Lithium
Salts. SEP. Lithium
Salts (sepwin.ch)
Paradox
basin brine project. US. Sheila Barradas. Creamer Media’s Mining Weekly. May
12. 2023. Paradox
basin brine project. US (miningweekly.com)
Utica
Shale Play Oil and Gas Brines: Geochemistry and Factors Influencing Wastewater
Management. Madalyn S. Blondes, Jenna L. Shelton, Mark A. Engle, Jason P.
Trembly, Colin A. Doolan, Aaron M. Jubb, Jessica C. Chenault, Elisabeth L.
Rowan, Ralph J. Haefner, and Brian E. Mailot. Environ. Sci. Technol. 2020, 54,
21, 13917–13925. October 14, 2020. Utica Shale Play Oil
and Gas Brines: Geochemistry and Factors Influencing Wastewater Management |
Environmental Science & Technology (acs.org)
Berkeley
Lab leading investigation to quantify and characterize Salton Sea’s geothermal
lithium resources. Green Car Congress. February 17, 2022. Berkeley
Lab leading investigation to quantify and characterize Salton Sea’s geothermal
lithium resources - Green Car Congress
Iodine
and Natural Gas. Nippoh Chemicals. Iodine and Natural Gas
| Unique Technologies | NIPPOH CHEMICALS (npckk.co.jp)
Iodine
Chemistry and Applications, Editor: Tatsuo Kaiho, Wiley, October 2014. Chapter
13: Iodine Production from Natural Gas Brine. Iodine
Production from Natural Gas Brine - Iodine Chemistry and Applications - Wiley
Online Library







































No comments:
Post a Comment